LED/UV Light-Curing Equipment and Accessories
LED/UV/Visible light-curing equipment for manufacturing. Dymax portfolio consists of spot lamps, flood lamps/focused beam, conveyor systems, and accessories.

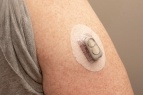
Designed for the assembly of wearable medical devices, the 2000-MW series is a dedicated light-curable, medical-grade adhesive product line specifically formulated without isobornyl acrylate (IBOA), a known skin irritant, and TPO, a material of concern. These first-in-class biocompatible wearable adhesives were developed to address changing customer requirements and skin sensitivity and proximity issues often associated with the medical wearables market.
This range of skin-friendly UV/LED light-curable adhesives, coatings, and encapsulants passed ISO 10993-10 / -23 for sensitization and irritation, as well as ISO 10993-5 for cytotoxicity. Designed for bonding SS, PC, PI, PVC, TPU, and difficult and low-surface-energy substrates, the adhesives are engineered for reliability with excellent adhesion and aging performance.
The products are used for bonding, sealing, encapsulating, and coating components in pain management, drug delivery, and vital sign and sleep monitoring medical wearable devices. Although Dymax medical wearable adhesives are not intended to be in direct contact with the skin, the 2000-MW series is intentionally designed to address concerns around skin sensitivity in the event of indirect or prolonged device contact.
In addition to the 2000-MW series of adhesives, Dymax also offers select medical-grade coating and adhesive products for medical electronics and device assembly. These products are intended for applications where skin sensitivity is less of a concern but biocompatibility is still preferred. Only products designated with “MW” in the product name are designed to be IBOA-free and tested for irritation and sensitization as well as formulated without TPO.
With wearable medical devices' constant proximity to skin, biocompatibility is a must. Our MW-series adhesives meet strict industry standards, passing ISO 10993 for cytotoxicity, sensitization, and irritation. They are also formulated without IBOA to be friendlier to skin, and without TPO or solvents for a safer product, workplace and environment.
Product Number
Product Description
Regional Availability



Product Number
Product Description
Regional Availability
Use our formulated product finder to help you find the right material. Interested in learning more or have questions? Contact Us, we want to hear from you.