Adhesives Formulated without IBOA
Skin is the largest organ in the human body. It breathes, regenerates, and is affected by what’s put on it. As wearable devices continue to grow in popularity the materials used to assemble, and in certain cases affix to the body, need to be taken into consideration.
The use of diagnostic and therapeutic medical wearable devices is becoming mainstream as the technology evolves and consumers demand simple ways of managing their health through monitoring, drug delivery, and pain management systems. Measurement tools like vital sign monitoring devices, sleep monitors, and continuous glucose monitors collect users’ exercise or personal health data, making it easy to keep track of the information, or even send it to a healthcare provider. Insulin pumps and wearable injectors offer a means of insulin and novel drug delivery in a non-clinical setting. These devices need to withstand repeated use, extended time on the body, exposure to different environments and be able to stay in place.
Along with adhesives, coatings, and encapsulants that are used to join and protect electronic components inside wearables, these materials are also used to join or seal the plastics and metals within the assembly. Additionally, adhesives can be used to attach devices to or be in contact with the skin for temporary or prolonged periods.
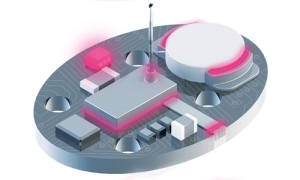
Medical wearable device diagram showing the areas where Dymax adhesive can be used to assemble components.
Sometimes the chemical IBOA (isobornyl acrylate) is found in wearables, which is a known skin sensitizer. IBOA, a monofunctional reactive diluent, is a critical monomer used in the manufacture of acrylic resins because it polymerizes when exposed to free radicals from an energy source such as UV light. IBOA is also used as a plasticizer within various medical device plastics due to its impact resistance, flexibility, and hardness qualities. If an adhesive or a substrate is formulated to include IBOA, it has the potential to cause a skin sensitivity issue if not fully cured and crosslinked.
Furthermore, adhesives, encapsulants, and coatings used to bond components inside a wearable device can potentially outgas during cure and redeposit onto other surfaces that may contact skin. If there is any “spillover” outside the adhesive channel, a small drop or section could be exposed externally. The uncured adhesive in an application can also cause similar issues.
As a result, users may experience skin sensitivity when using medical wearables.
For these reasons, it’s important for manufacturers involved in the design and assembly of wearable devices to use adhesives that are formulated with skin sensitivity in mind. Adhesives must be formulated without IBOA and have passed ISO 10993 evaluations for irritation and sensitization. Other criteria to consider are ISO 10993-5 Cytotoxicity biocompatibility, the ability to adhere to low-surface-energy substrates and to be moisture and thermal-shock resistant.
Dymax manufactures IBOA-free light-curable adhesives for medical wearable device assembly that meet the ISO 10993-10 standard and that verifies they are not skin sensitizers when fully cured.
To learn more about this line of materials or to discuss your application, contact our Application Engineering team.
_________________________________________________________
Enjoying This Content? Let’s Stay Connected.
If you’re finding value in our insights, why not get more of it—delivered right to your inbox? Subscribe to receive the latest technical articles, white papers, product news, and expert tips.